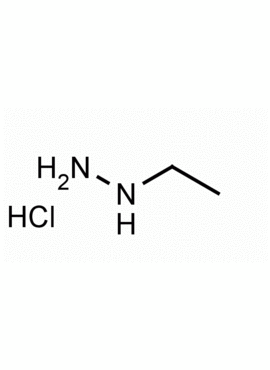
1-Ethylhydrazine hydrochloride CAS: 18413-14-4 MDL: MFCD01722460
Molecular weight: 96.56 g/mol
Molecular Formula: C2H9ClN2
CAS Number: 18413-14-4
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: Ethylhydrazine hydrochloride, 18413-14-4, Hydrazine, ethyl-, monohydrochloride, 1-Ethylhydrazine hydrochloride, Ethylhydrazine.HCl
Uses: Reactive reagent, protein labelling, active site labels, active site mapping, pyrazole synthesis, pharmaceutical synthesis, medicinal chemistry of pyrazole
1-Ethylhydrazine hydrochloride, is a synthetic fine chemical useful for studying the biochemistry of macromolecules.
Selected References:
Aiello, E., S. Aiello, F. Mingoia, A. Bacchi, G. Pelizzi, C. Musiu, M. Giovanna Setzu, A. Pani, P. La Colla, and M. Elena Marongiu. Synthesis and Antimicrobial Activity of New 3-(1-R-3(5)-Methyl-4-Nitroso-1H-5(3)-Pyrazolyl)-5-Methylisoxazoles. Bioorganic & Medicinal Chemistry 8, (2000): 2719–28. https://doi.org/10.1016/S0968-0896(00)00211-X.
Antonini, Ippolito, Paolo Polucci, Amelia Magnano, and Sante. Martelli. Synthesis, Antitumor Cytotoxicity, and DNA-Binding of Novel N-5,2-Di(ω-Aminoalkyl)-2,6-Dihydropyrazolo[3,4,5-Kl]Acridine-5-Carboxamides. Journal of Medicinal Chemistry 44, (2001): 3329–33. https://doi.org/10.1021/jm010917o.
Ator, Mark A., Shantha K. David, and Paul R. Ortiz de Montellano. Stabilized Isoporphyrin Intermediates in the Inactivation of Horseradish Peroxidase by Alkylhydrazines. Journal of Biological Chemistry 264, (1989): 9250–57.
Calle, Mariola, Luis A. Calvo, Alfonso Gonzalez-Ortega, and Ana M. Gonzalez-Nogal. Silylated β-Enaminones as Precursors in the Regioselective Synthesis of Silyl Pyrazoles. Tetrahedron 62, (2006): 611–18. https://doi.org/10.1016/j.tet.2005.10.001.
Curran, Kevin J., Jeroen C. Verheijen, Joshua Kaplan, David J. Richard, Lourdes Toral-Barza, Irwin Hollander, Judy Lucas, Semiramis Ayral-Kaloustian, Ker Yu, and Arie. Zask. Pyrazolopyrimidines as Highly Potent and Selective, ATP-Competitive Inhibitors of the Mammalian Target of Rapamycin (MTOR): Optimization of the 1-Substituent. Bioorganic & Medicinal Chemistry Letters 20, (2010): 1440–44. https://doi.org/10.1016/j.bmcl.2009.12.086.
McLeod, Matt, Nicolas Boudreault, and Yves. Leblanc. Synthetic Application of Monoprotected Hydrazines toward the Synthesis of 1-Aminopyrroles. Journal of Organic Chemistry 61, (1996): 1180–83. https://doi.org/10.1021/JO9518260.
Steffan, Robert J., Edward Matelan, Mark A. Ashwell, William J. Moore, William R. Solvibile, Eugene Trybulski, Christopher C. Chadwick, et al. Synthesis and Activity of Substituted 4-(Indazol-3-Yl)Phenols as Pathway-Selective Estrogen Receptor Ligands Useful in the Treatment of Rheumatoid Arthritis. Journal of Medicinal Chemistry 47, (2004): 6435–38. https://doi.org/10.1021/jm049194+.