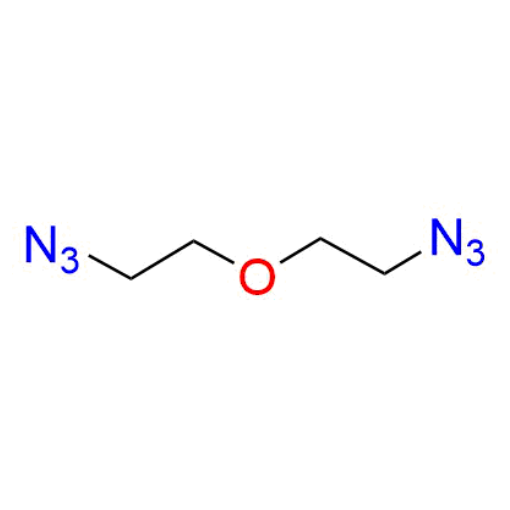
Name: 1-azido-2-(2-azidoethoxy)ethane (98%)
Molecular Formula: C4H8N6O
CAS#: 24345-74-2
SMILES: [N-]=[N+]=NCCOCCN=[N+]=[N-]
MDL#: MFCD11655885
Catalog#: AMTGC779-AA21
Molecular weight: 156.08 g/mol
Other names:
- 1,5-diazido-3-oxapentane
Fields of Interest: PEGylation, click chemistry, crosslinking, bioconjugation, materials science
Background & Applications:
Background
1-Azido-2-(2-azidoethoxy)ethane (CAS 24345-74-2) is a compact, symmetrical diazide-functionalized PEG derivative based on a PEG2 backbone, featuring azide groups at both termini. Despite its short chain length, the polyethylene glycol spacer provides improved hydrophilicity and flexibility compared to purely aliphatic diazides, while maintaining minimal spacing. The terminal azide functionalities enable highly efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition, allowing simultaneous or stepwise conjugation at both ends. This small bifunctional linker is a valuable building block within a versatile portfolio of functionalized PEGs designed for controlled molecular coupling.
Applications
1-Azido-2-(2-azidoethoxy)ethane is commonly used in crosslinking, bioconjugation, and materials science applications where short linker length and dual click reactivity are required. Typical uses include construction of compact PEG-based networks, preparation of multifunctional intermediates, and surface or nanoparticle modification via dual azide coupling. As part of a comprehensive functionalized PEG product line, this PEG2 diazide supports modular design strategies in pharmaceutical research, diagnostics, biomaterials, and advanced materials development where tight spatial control is critical.
Appearance: Pale yellow liquid
Purity: 98%
Storage: 0-3 °C for long term storage
Solubility: DCM, Chloroform, MeOH, EtOH
Literature:
- European Journal of Organic Chemistry, 2015, vol. 2015, 29, p. 6458 – 6465.
- Tetrahedron, 1992, vol. 48, 8, p. 1497 – 1506