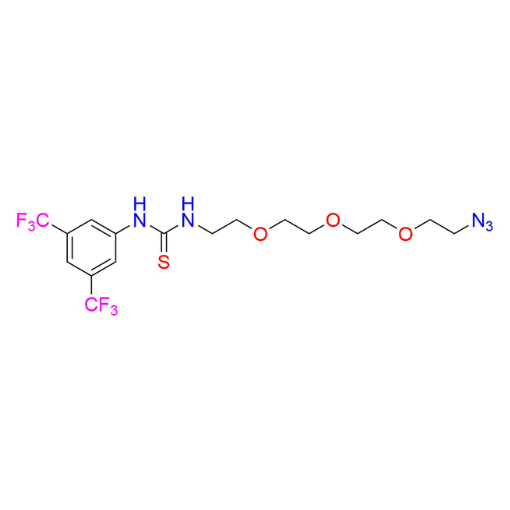
Name: 1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea
Molecular Formula: C17H21F6N5O3S
CAS#: 2185837-85-6
SMILES: S=C(NC1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1)NCCOCCOCCOCCN=[N+]=[N-]
MDL#: none found
Catalog#: AMTGC1282-TF24
Molecular weight: 489.44g/mol
Other names:
- Azido-PEG4-thiourea (3,5-bis(trifluoromethyl)phenyl)
- PEG4-azide-linked 3,5-bis(trifluoromethyl)phenyl thiourea
- PEG4-azide-linked aryl thiourea
- N-(PEG4-azidoethyl)-N′-(3,5-bis(trifluoromethyl)phenyl)thiourea
Fields of Interest: PEGylation, bioconjugation, materials science, chemical probe development, click chemistry
Background & Applications:
Background
1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea (CAS 2185837-85-6) is an azide-functionalized PEG conjugate featuring a PEG4 spacer that links a terminal azide group to a thiourea-bearing aromatic moiety substituted with trifluoromethyl groups. The polyethylene glycol chain provides hydrophilicity, flexibility, and improved solubility, while spatially separating the aromatic thiourea functionality from the reactive azide. The azide group enables efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition, allowing site-specific attachment to alkynyl-functionalized molecules or surfaces. This compound extends the scope of functionalized PEGs by integrating a chemically and biologically relevant thiourea motif.
Applications
This azido-PEG-thiourea derivative is used in bioconjugation, materials science, and chemical probe development applications where controlled presentation of a thiourea-containing aromatic group is required. Typical uses include click-based attachment of the PEG–thiourea unit to polymers, surfaces, or biomolecules, preparation of functional probes and intermediates, and incorporation into advanced materials for binding, sensing, or mechanistic studies. As part of a comprehensive functionalized PEG product line, 1-(2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea supports modular design strategies that combine PEG solubility with specialized functional groups for research and specialty chemical applications.
Appearance: Pale yellow viscous oil
Purity: 99%
Storage: 0-3 °C for long term storage
Solubility: DCM, Chloroform, Ethyl Acetate
Literature:
The thiourea motif featuring the 3,5-bis(trifluoromethyl)phenyl group — part of this compound’s structure — is well represented in the literature as a key structural unit in organocatalysis and (thio)urea hydrogen-bond catalysis. See this review on related thiourea catalysts in organic reactions:
N,N′‑bis[3,5‑bis(trifluoromethyl)phenyl]thiourea: a privileged motif for catalyst development
This paper discusses how the 3,5-bis(trifluoromethyl)phenyl thiourea scaffold has been used to activate substrates and stabilize transition states in organic synthesis.