Frontier Specialty Chemicals has extensive experience in the field of Organoborons and derivatives. Frontier offered the first commercially available bis(pinacol)diboron to the synthetic community.
Boronic acids, along with their corresponding boronate esters and potassium trifluoroborate salts, have long been used in synthetic organic chemistry to construct C-C and C-heteroatom bonds as well as C-H functionalization via metal-mediated cross coupling reactions. Thus, boronic acids are very valuable to synthetic chemists as building blocks and key intermediates in the synthesis of more complex molecules and also play important roles in medicinal chemistry and chemical biology.
Boronic acids and derivatives with their air, moisture, thermal stability, functional group tolerance, and low toxicity, along with commercial availability have made them ideally suited for coupling purposes.
Boronic Acids as Synthetic Building Blocks
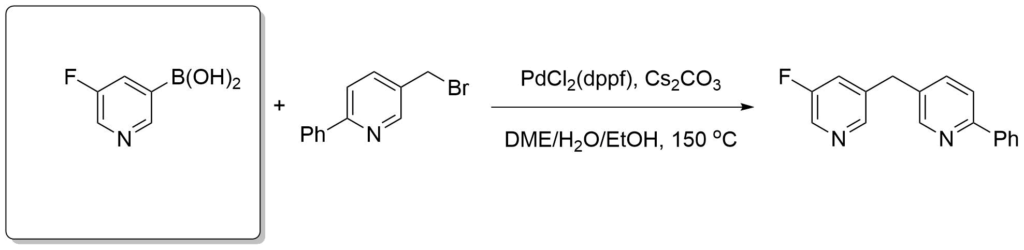
Emmerich, J. et al., J. Med. Chem. 2013, 56(15), 6022-6032.
In general, boronate esters are highly air and water stable and are very resistant to hydrolysis. They are less polar than boronic acids and are generally easier to handle, purify, and characterize. Unlike boronic acids they do not form anhydrides. These characters can make boronate esters a preferable alternative choice to boronic acids in some synthetic applications.
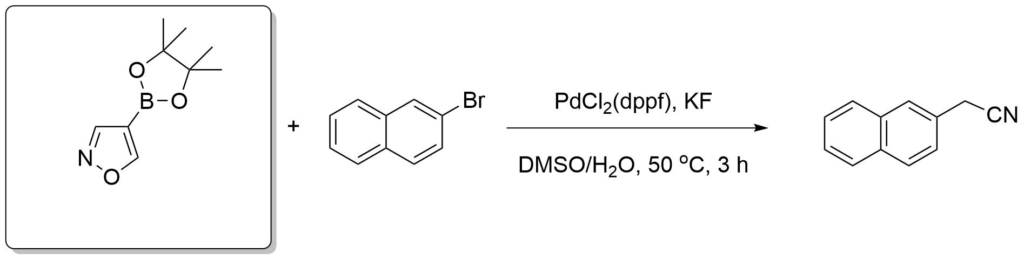
Velcicky, J. et al., J. Am. Chem. Soc., 2011, 133(18), 6948-6951.
Suzuki-Miyaura coupling allows for the coupling of boronic acids and their derivatives typically with carbon halides and triflates. The cross-coupling of the catalytic cycle begins with oxidative addition, then the transmetalation and ends with reductive elimination. A general pathway of metal mediated Suzuki-Miyaura cross-coupling catalytic cycle is illustrated in the following scheme between an organic halide and boronic acid with palladium catalyst.
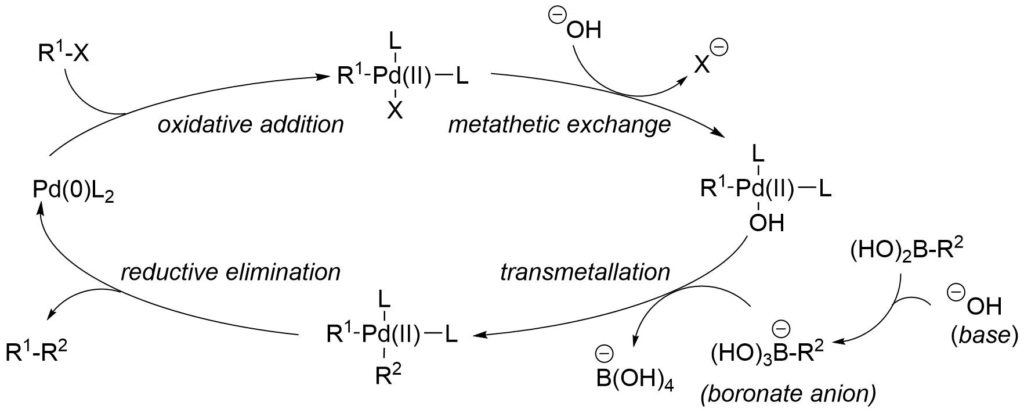
Suzuki-Miyaura Cross-Coupling Catalytic Cycle
Potassium trifluoroborate salts of boronic acids are also versatile reagents in organic synthesis. They overcome some limitations of the electrophilic boron atom and can be used in transition-metal-catalyzed reactions to introduce a wide range of groups including, alkyl, alkenyl, alkynyl, aryl, allyl, etc. The organotrifluoroborates are crystalline solids or free flowing powders and the stability of tetracoordinated boron minimizes decomposition pathways such as protodeboronation in storage. This distinctive reactivity complements those of tricoordinate organoborons and permits nucleophiles to be utilized as reactants toward electrophilic functional groups incorporated within the organoboron compounds themselves.
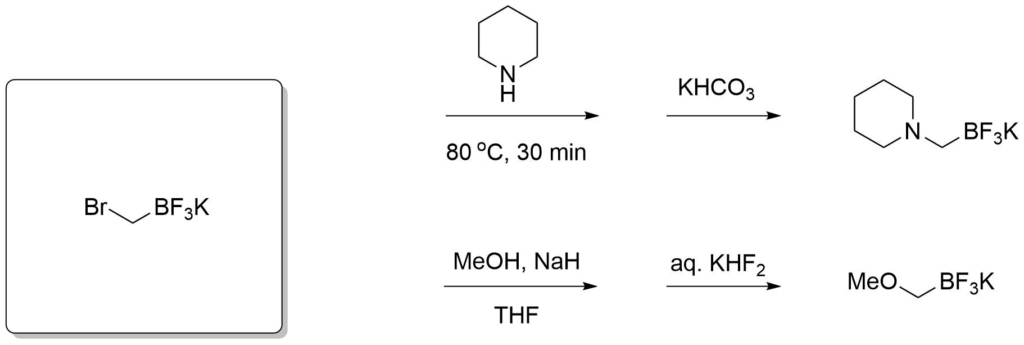
Molander, G. A. et al., Org. Lett., 2006, 8(10), 2031-2034
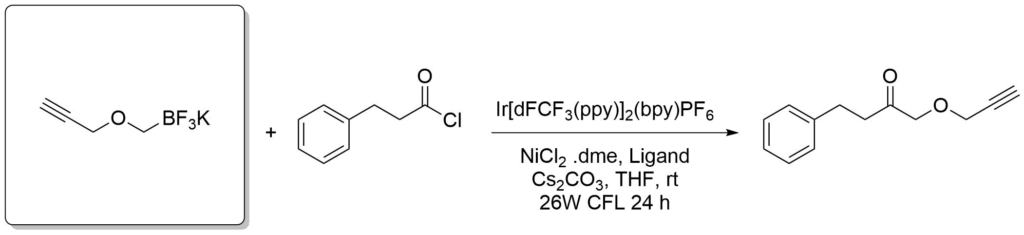
Amani, J. et al., Org. Lett., 2016, 18(4), 732-735.
Boronic acids as catalysts
Boronic acids can be used to activate carboxylic acids towards attack by nucleophiles, and the activation of hydroxyl groups.
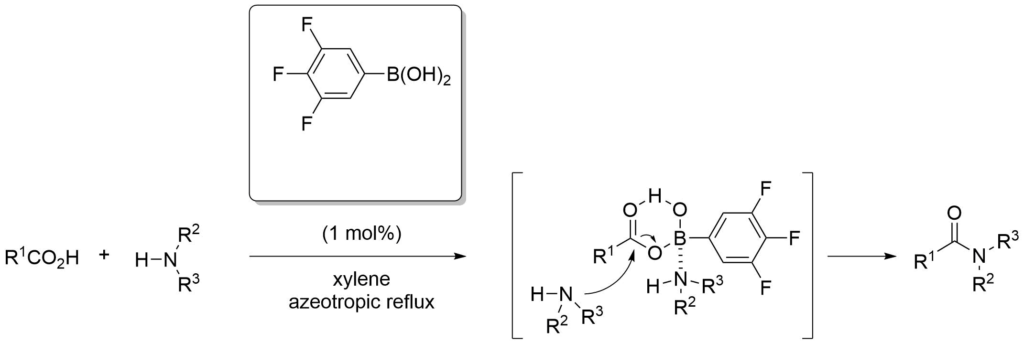
Ishihara, K. et al., J. Org. Chem., (1996), 61, 4196-4197.
Boronic Acids in Pharmaceutical Applications
Proteasome inhibitors for the treatment of multiple myeloma.
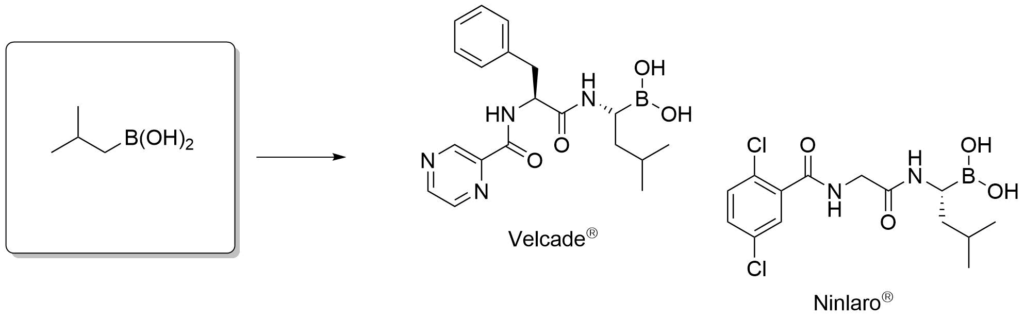
Adams, J. et al., Cancer Invest., 2004, 22, 304-311.
Avet-Loiseau. et al., Blood, 2017, 130, 2610-2618.
Antifungal agents for the treatment of eczema.
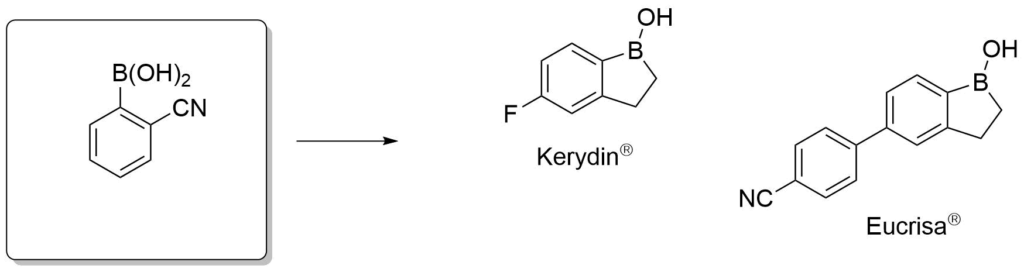
Elewski, B. E. et al., J. Am. Acad. Dermatol., 2015, 73, 62-69.
Akama, T. et al., Bioorg. Med. Chem. Lett., 2009, 19, 2129-2132.
Boronic Acids in MRI Visualization
In-vivo tumor targeting based on the recognition of over-expressed sialic acid by the pba-based imaging reporter.
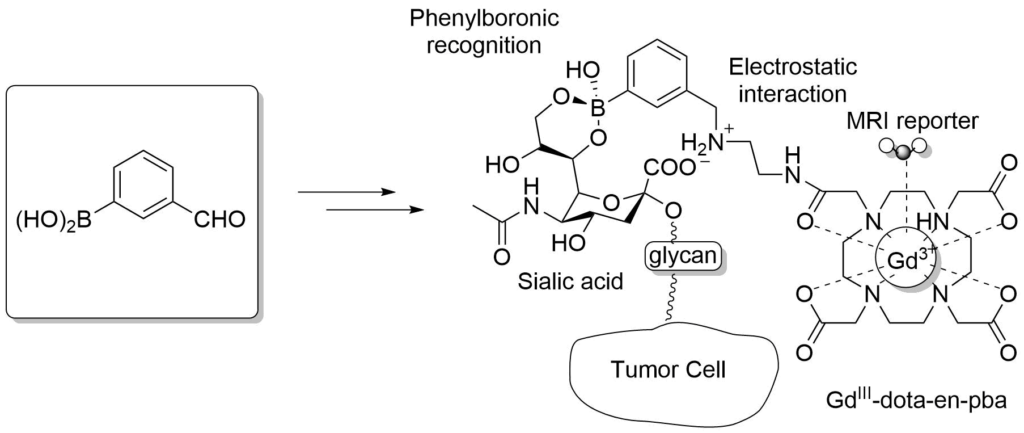
Crich, S. G. et al., Angew. Chem. Int. Ed. 2013, 52, 1161-1164.
References
- Miyaura, N., Suzuki, A. Chem Rev. 1995, 95, 2457-2483.
- Suzuki, A., Brown, H. C. “Organic Syntheses via Boranes, Volume 3 Suzuki Coupling” Aldrich Chemical Co., Milwaukee, WI 2003.
- Hall, G. H. “Boronic acids” 2nd Ed. Weinheim, Germany: Wiley-VCH Verlag & Co. (2011) (Vols. 1-2).
- Molander, G. A. “Handbook of Reagents for Organic Synthesis: Catalyst Components for Coupling Reactions”. West Sussex, England: John Wiley & Sons. 2008.
- Emmerich, J. et al., J. Med. Chem. 2013, 56(15), 6022-6032.
- Velcicky, J. et al., J. Am. Chem. Soc., 2011, 133(18), 6948-6951.
- Molander, G. A. et al., Org. Lett., 2006, 8(10), 2031-2034; Org. Lett., 10(11), 2135-2138.
- Amani, J. et al., Org. Lett., 2016, 18(4), 732-735.
- Ishihara, K. et al., J. Org. Chem., (1996), 61, 4196-4197.
- Adams, J., et al. Cancer Invest., 2004, 22, 304-311.
- Avet-Loiseau., et al. Blood, 2017, 130, 2610-2618.
- Elewski, B. E., et al. J. Am. Acad. Dermatol., 2015, 73, 62-69.
- Akama, T., et al. Bioorg, Med. Chem. Lett., 2009, 19, 2129-2132.
- Crich, S. G. et al. Angew. Chem. Int. Ed. 2013, 52, 1161-1164.